Having optimised a reproducible process for preparing Polystyrene Sulfonic Acid (PSSA) from waste Polystyrene (PS), we were keen to test its catalytic activity. The sample shown in the video has a sulfonation degree of 44%, determined by titration with NaOH using phenolphthalein as an indicator. This corresponds to 2.37 mmol of H⁺ per gram of PSSA.
In our MSc Sustainable Chemistry programme at UCL, we focus on using biobased and platform chemicals derived from biomass for the synthesis of valuable organic molecules. At the time we developed the protocol for obtaining PSSA, one of our MSc research projects was investigating solid acid-catalysed reactions to produce biodiesel precursors. We decided to test our PSSA catalyst in the synthesis of biodiesel precursor A, using the biobased chemicals furfural and 2-methylfuran. Precursor A is just one hydrogenation step away from a biodiesel molecule.
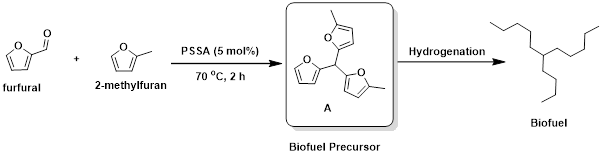
The reaction performed very well on a 0.5 mmol scale of furfural, using 0.5 mL of 2-methylfuran as both solvent and reactant. With 5 mol% of PSSA catalyst, the reaction was complete within 2 hours, yielding the product quantitatively. Since PSSA is insoluble, it can be easily recovered by vacuum filtration after the reaction (see Experimental Section).

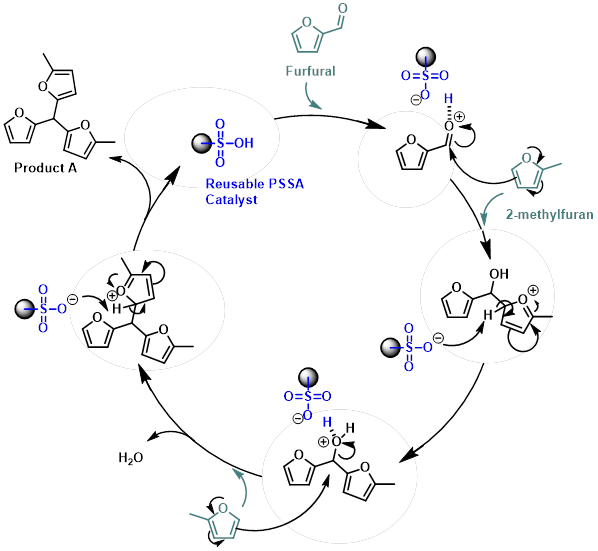
The reaction between furfural and 2-methylfuran to form biofuel precursor A follows a Friedel–Crafts-type mechanism, as illustrated below. Product A has previously been described in the literature as a colourless oil when purified by distillation. We typically obtain a faint pink to red oil, which we suspect may result from trace acid contamination during silica gel purification. Furan rings are known to be sensitive to acidic conditions. Nevertheless, our purified samples appear to be of high purity based on NMR analysis.
This successful application of PSSA derived from waste PS in organic synthesis encourages us to continue developing further examples. We aim to contribute to the literature and inspire others interested in upcycling plastic waste from laboratories into valuable chemical tools.