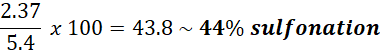An approximation of the degree of sulfonation of polystyrene sulfonic acid (PSSA) synthesised from PS waste can be calculated by determining the number of acidic protons from the sulfonic acid (–SO₃H) groups present in the polymer. While this method provides a useful estimate, the precise sulfur content should ideally be determined by elemental analysis. Nevertheless, calculating the number of active acid sites allows us to evaluate the catalytic potential of PSSA in organic synthesis.
The degree of sulfonation is determined by titrating a sample of PSSA with a 0.01 M NaOH solution in water, using phenolphthalein as an indicator. Since PSSA is not soluble in water, the titration is performed as a suspension. The colour change can be slow and may lead to false positives, so the titration should only be considered complete if the pink colour (typical of phenolphthalein in basic solution) persists for 15–20 minutes. For better visibility, it is recommended to place a piece of white paper under the conical flask during titration.

To calculate the degree of sulfonation, we consider the mass of the repeating unit of a fully para-sulfonated polystyrene resin. The molar mass of this unit is 184.02 g/mol, meaning that 1 g of fully sulfonated PS contains:
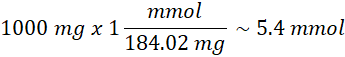
Meaning that a fully sulfonated resin has 5.4 mmol of H+ in 1g of resin.

In a typical protocol, 20 mg of PSSA are suspended in 20 mL of distilled water in a small conical flask. Two drops of phenolphthalein solution are added as an indicator, and the resin is titrated with 0.01 M NaOH. If the titration endpoint is reached at 4.75 mL of NaOH, the degree of sulfonation is calculated as:
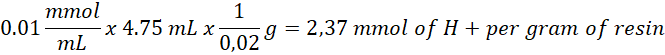
Given the theoretical maximum of 5.4 mmol/g, the percentage of sulfonation is: