Polystyrene sulfonic acid (PSSA) resins are commercially available and can be synthesised from polystyrene (PS) via direct sulfonation, a classic textbook reaction familiar to many chemistry students (Fig. 1).
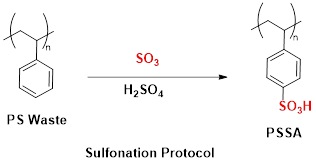
Fig 1. Sulfonation of PS with concentrated sulfuric acid
However, many sulfonation protocols described in the literature lack sufficient detail, and the isolation or purification of PSSA often relies on complex equipment that may not be readily available or affordable in typical lab settings. As a result, using sulfonation to upcycle single-use PS waste generated in the lab can be quite challenging.
One of the first issues we encountered was our limited experience working with polymers. We chose waste expanded polystyrene as our substrate and tested various sulfonation protocols until we identified reproducible conditions that allowed us to isolate PSSA effectively.
Working with expanded PS is surprisingly frustrating. A small mass occupies a large volume, and when you try to cut it into smaller pieces, it becomes statically charged and sticks to everything, except your flask, creating a mess. These are the kinds of practical issues rarely mentioned in the literature.
Fortunately, PS is highly soluble in several organic solvents. It dissolves readily in small amounts of dichloromethane (DCM), but we recommend using a greener alternative like ethyl acetate, which also dissolves PS efficiently. Once dissolved and the solvent is evaporated, the PS occupies significantly less volume, making it much easier to handle.

Fig 2. A) Volume of 1 g of expanded PS in a 400 mL beaker. B) Volume of the same amount of PS after dissolution in ethyl acetate and solvent evaporation
We found that optimal sulfonation conditions involved reacting 1 g of PS with 10 mL of concentrated sulfuric acid at 100 °C for 1.5 hours. While conditions vary in the literature, we observed that extending the reaction to 2 hours increases the degree of sulfonation and homogeneity but also makes isolation more difficult using standard lab equipment. Some published methods rely on sophisticated membranes and dialysis techniques, which are not accessible in every lab. The goal of the RECOMPENSE project has always been to develop a process that is simple, affordable, and user-friendly.
Using our standard conditions (see video and experimental section), we obtained PSSA samples with a degree of sulfonation ranging from 30% to 46%, measured as H⁺ per gram of resin (see our titration post for details). Once the reaction is repeated under consistent conditions, results become more reproducible. Variability is usually due to incomplete contact between reduced PS and sulfuric acid.
Our isolation protocol is straightforward: dilute the reaction mixture with water (carefully, using an ice-water bath), which precipitates PSSA as an insoluble mass. This can be filtered and washed repeatedly with water until the filtrate is neutral. Finally, dry the PSSA in an oven at 70 °C for 24 hours to obtain a yellowish-white powder. The resulting PSSA is mostly insoluble in deuterated solvents but can be characterised by IR spectroscopy and titration.

Fig 3. Appearance of an isolated sample of PSSA obtained from waste expanded PS
FTIR analysis (Fig. 4) of our PSSA sample reveals a new broad band at 3405 cm⁻¹, corresponding to O–H stretching vibrations associated with sulfonic acid (–SO₃H) groups. Additionally, the spectrum shows distinctive bands in the 1000–1200 cm⁻¹ region, which are characteristic of sulfonate stretching vibrations, absent in the original waste PS sample.

Fig 4. A) FTIR spectrum of a waste expanded PS sample. B) FTIR spectrum of our PSSA sample from waste PS featuring a new O-H stretching band at 3405 cm-1 from the sulfonic acid functional group and sulfonate stretch bands between 1000 and 1200 cm-1.
Watch our next video to learn how to determine the number of acidic sites in your PSSA using a simple titration with NaOH and phenolphthalein. Once you know the amount of acidic sites per gram, you are ready to use your upcycled PSSA as a solid acid catalyst in organic synthesis.

